Today was Signature Day! All of the STEM interns and Signature creators presented their projects to a crowd of students, faculty, grandparents, and special friends. It was a lot of fun to show everyone the results of my research and help them understand my project.
I presented at 9:00 in the morning to a large crowd crammed into Snell 102. After Nana and Michaela finished summing up their projects, I got up to give my presentation. Everything went smoothly, and I got a lot of great questions afterward!
This entire year has been incredible. I have loved working at RPI in the CBIS High School Scholars Program. Yi and Dr. Royer are both brilliant and kind people, and their guidance and wisdom have really enriched my senior year.
If any Emma students (current or prospective) are reading this post, I hope you strongly consider doing a STEM internship as soon as you are able to. It was so much fun to obtain so much knowledge and lab experience. It definitely made senior year much more enjoyable!
Thank you to everyone who has helped me move forward with this project. I want to give particular mention to Yi, Dr. Royer, Ms. Mossop, Ms. Biggins, Mr. Calos, the shuttle drivers and to all the people who made little contributions to my experience along the way. Thank you!
Molly's Internship at RPI
Friday, May 19, 2017
Tuesday, April 25, 2017
Week of 4/23/17
Today was prep for my poster session, which is this Thursday. I am very excited to present at RPI as part of the CBIS High School Scholars Program. Rather than describe my preparations, I thought I would post my notes here for you to read and give an idea of what I am going to say this Thursday when I present.
---------------------------------------------------------------------------------------------------------------------
Protein folding is one of the most important biological
processes
For many proteins to perform their biological processes,
they must fold into a specific 3D structure
This process is called protein folding – are interested in
how folding processes throughout this process
If we want to study protein folding, we need to perturb this
process – otherwise it is stuck at equilibrium
There are sever types of perturbants
Chemical denaturants (urea, guanidine hydrochloride)
Temperature, changing pH
But in our lab, we use pressure
Talk about how pressure perturbs folding – due to internal
cavities in internal structure
In the folded protein, packing is imperfect
In folded structure, due to imperfect packing of atoms,
there are solvent excluded cavities (where water cannot enter)
Pressure acts to eliminate these cavities (bc pressure
minimizes volume) and unfold the protein
From this pressure study, we can obtain volumetric
properties of the protein
Pressure only acts on the cavities – cavities are different
for each protein – this way, pressure perturbs cavities of different proteins
differentially
Pressure effect has local feature, therefore it reveals more
detailed information regarding the protein folding process
Why not chemical? Temperature?
Chemical has global effect
Protein we study: PP32
PP32 belongs to acidic nuclear phospho protein family
Tumor suppressor – good for cancer research (biological
significance)
We’re more about physical significance
Leucine-rich pp protein
Repeat protein (5 repeats – arrows) conserves leucine
residues within repeats
Repeats are similar in structure and sequence (look similar
– leucine rich at one part of arrow? Same
for next arrow)
Cavity in center of structure (empty – no water) spheres
We study this protein because it is a repeat protein so its
overall architecture is linear and simple – easy to study
Simple linear architecture abundant in local contact,
lacking global interaction (by folding studies)
Protein folding is matter of structural energetic
interaction
Also good for NMR – use fluorescence (tryptophan, terasine,
phenylalanine)
Only rings can accept fluoresce
Quantum yield – how much light is emitted (give 10 photons,
how may do you get back)
Tryptophan has higher quantum yields than other two – so low
we can’t see it
Unfortunately, there is no tryptophan in this protein
Tryptophan residue introduced at c terminus for fluorescence
measurements
Yellow c terminus
PP32 has two capping domains – on two termini (stabilize
protein)
Two black sticks are two residues: aspartic acid 146 and
tyrosine 131
Numbers are residue number (area around first top loop is
approx. 18)
Why are they there? Side chains close to one another- hydrogen bonding between the two
Keeps the structure stable and stabilizes the entire protein
(there naturally)
How the fluorescence works
Tryptophan likes to be excited with wavelength 290
nanometers
Gives emission spectrum which are dependent on micro
environment around that residue
Environment is determined by protein structure
Therefore when proteins are folded and unfolded, we get
different emission spectra (different
environment)
Folded is lower peak –
Usually, folded has higher peak, but in this case – we think
there are histanine residues around the
tryptophan which quenches the fluorescence
(when it is folded) – when protein unfolds, quenching effect dissipates
Folded and unfolded states have different emission spectra –
can be differentiated
With the mutations (change Y131 to F and D146 to L) – we can
break up the hydrogen bond and unfold the protein
Take fluorescence measurement, increase pressure to new
level, protein unfolds to some degree, let it
reach new equilibrium, take next
fluorescence measurement, repeat
That’s how the instrument works -> water pumped in and
increases pressure
For each measurement, we increase the pressure and protein
starts to unfold -> takes time to unfold
Excitation wavelength at 290 nm
In fact, when Yi increased pressure, he didn’t take full
emission spectra – takes too long
Just one – 340 nm
Averaged equilibrium values for intensity graph
Protein dissolved in urea (helps to unfold), water, bis-tris
(pH buffer) – more denaturant added, easier for pressure to unfold protein, DTT
(reducing agent – prevents proteins from aggregating)
Urea facilitates protein unfolding
pH 6.8 20 degrees
What we did: took emission spectra of this protein after the
system reaches equilibrium
How do you know it reached equilibrium?
Monitored intensity at 340 nm as function of time ->
signal doesn’t change anymore
Take intensity at 340 nm and plotted it as a function of
pressure – going past 340, intensity is the same (asymptote)
At each pressure, take the value at 30 nm -> plot 340 as
function of pressure
Sigmoidal (s curve)
Unfolded – higher value
Folded – lower value
Two state model – looks at unfolded versus folded (we follow two state mode to analyze data)
Transition state is population weighted average of two
states – percent unfolded and percent folded average gives transitional value
Delta g value varies with amount of urea
---------------------------------------------------------------------------------------------------------------------
I hope my notes are somewhat intelligible. I am very excited to present my poster!
Monday, April 3, 2017
Week of 4/2/17
Today was my first day back in the lab in over a month! I was really eager to get back. Yi finished his thesis defense, which is awesome, but that also meant that e was really tired so we ended up just doing a few simple tasks in the lab today.
First, we prepared some cultures and began growing bacteria. I pipetted three milliliters of LB broth into a tube under a flame, and repeated this process five times. Then, Yi gave me six different samples of bacteria to pipette into the individual tubes. Again, we performed this process under the flame.
Once all of the bacteria had been loaded into the tubes with growth medium and labeled, we placed them in the fridge to grow. If we had wanted them to grow faster, we could have put them in an incubator shaker for two to three hours at thirty-seven degrees. When bacterial samples are shook, the agitation incorporates oxygen and evenly distributes nutrients to all the bacteria, helping promote faster growth.

An incubator shaker. https://megadepot.com/product/ika-works-3940100-ks-3000-ic-control-incubator-shaker
After leaving the bacteria in the fridge, Yi and I had no more work to do. We offered help to another graduate student working in the lab, and she asked us to prepare 250 milliliters of 3 M NaCl. It was quite simple to calculate how many grams of powdered NaCl to use, I have outlined my calculations below.
3 mol / L * (.250 L) = .75 mol NaCl wanted
.75 mol 8 (58.44 g / mol) = 43.83 grams of NaCl
I weighed 43.83 grams of NaCl and poured it into a liter flask. Then, I added enough distilled water to bring the total number of milliliters of solution to 250 mL, and then shook and swirled the flask vigorously until the solution was completely dissolved.
I was very happy to get back in the lab this week. Even the simplest tasks are really fun, and I appreciate them even more as the year winds down. Can't wait for next week!
First, we prepared some cultures and began growing bacteria. I pipetted three milliliters of LB broth into a tube under a flame, and repeated this process five times. Then, Yi gave me six different samples of bacteria to pipette into the individual tubes. Again, we performed this process under the flame.
Once all of the bacteria had been loaded into the tubes with growth medium and labeled, we placed them in the fridge to grow. If we had wanted them to grow faster, we could have put them in an incubator shaker for two to three hours at thirty-seven degrees. When bacterial samples are shook, the agitation incorporates oxygen and evenly distributes nutrients to all the bacteria, helping promote faster growth.

An incubator shaker. https://megadepot.com/product/ika-works-3940100-ks-3000-ic-control-incubator-shaker
After leaving the bacteria in the fridge, Yi and I had no more work to do. We offered help to another graduate student working in the lab, and she asked us to prepare 250 milliliters of 3 M NaCl. It was quite simple to calculate how many grams of powdered NaCl to use, I have outlined my calculations below.
3 mol / L * (.250 L) = .75 mol NaCl wanted
.75 mol 8 (58.44 g / mol) = 43.83 grams of NaCl
I weighed 43.83 grams of NaCl and poured it into a liter flask. Then, I added enough distilled water to bring the total number of milliliters of solution to 250 mL, and then shook and swirled the flask vigorously until the solution was completely dissolved.
I was very happy to get back in the lab this week. Even the simplest tasks are really fun, and I appreciate them even more as the year winds down. Can't wait for next week!
Friday, March 31, 2017
Week of 3/26/17
Yi had his thesis defense today, so I didn't end up going to RPI. I can't wait to get back!
Sunday, March 5, 2017
Week of 3/5/17
Yi had a meeting today so I couldn't get to RPI, but I eagerly anticipate our next session!
Wednesday, February 8, 2017
Week of 2/5/17
This week's meeting was great! Yi and I used the agar growth mediums we prepared a few weeks ago to plate the bacteria. We unwrapped the three petri dishes from their parafilm seals and set them aside. Next, we gathered the materials necessary for plating the bacteria- a sterile loop, Bunsen burner, and the bacteria culture.
Once we had all of our materials, it was time to plate the bacteria. We turned on the Bunsen burner and waved the loop quickly through the flame to ensure the surface was sterile. Making sure to keep all activity under the burner (again, to prevent contamination), we stuck the loop into the tube of bacteria and picked up a small quantity of bacteria. Then, we transferred the bacteria onto the agar plate using a criss-cross pattern, as shown below.
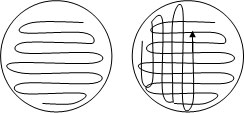
Bacteria on agar criss-cross pattern. http://teachersinstitute.yale.edu/curriculum/units/2010/3/10.03.01.x.html
Why were the bacteria plated in a criss-cross pattern? This is to ensure that the bacteria do not grow clumped and crowded with one another. This pattern not only ensures a more equal distribution of resources, but also makes it so that individual colonies can be observed as they grow, rather than a mass collection of colonies impossible to study.
It should also be noted that our bacteria culture included a small amount of antibiotic within its medium. The bacteria we were studying possessed resistance to the antibiotic, and thus were not affected by the drug. This again was a preventative measure against contamination- any bacteria that somehow enters the chamber will be killed by the antibiotic, ensuring that the only growing bacteria will be the desired strain of study.
By observing the growth patterns of our strain of bacteria, we can see the expansion of individual colonies. Although I have performed experiments like this before, it was great to be exposed to more lab techniques and practice scientific skills in a real laboratory setting. This meeting was great, and I cant wait for next week's internship meeting!
Once we had all of our materials, it was time to plate the bacteria. We turned on the Bunsen burner and waved the loop quickly through the flame to ensure the surface was sterile. Making sure to keep all activity under the burner (again, to prevent contamination), we stuck the loop into the tube of bacteria and picked up a small quantity of bacteria. Then, we transferred the bacteria onto the agar plate using a criss-cross pattern, as shown below.

Bacteria on agar criss-cross pattern. http://teachersinstitute.yale.edu/curriculum/units/2010/3/10.03.01.x.html
Why were the bacteria plated in a criss-cross pattern? This is to ensure that the bacteria do not grow clumped and crowded with one another. This pattern not only ensures a more equal distribution of resources, but also makes it so that individual colonies can be observed as they grow, rather than a mass collection of colonies impossible to study.
It should also be noted that our bacteria culture included a small amount of antibiotic within its medium. The bacteria we were studying possessed resistance to the antibiotic, and thus were not affected by the drug. This again was a preventative measure against contamination- any bacteria that somehow enters the chamber will be killed by the antibiotic, ensuring that the only growing bacteria will be the desired strain of study.
By observing the growth patterns of our strain of bacteria, we can see the expansion of individual colonies. Although I have performed experiments like this before, it was great to be exposed to more lab techniques and practice scientific skills in a real laboratory setting. This meeting was great, and I cant wait for next week's internship meeting!
Monday, January 30, 2017
Week of 1/30/17
Time for the interview! After working in the lab and placing our bacteria in the growth medium that we prepared last week, Yi and I sat down for a few minutes so that I could ask him some questions. Enjoy!
Great meeting this week, and Yi really provided some valuable knowledge and insight. I can't wait to get back in the lab next week!
Interviewer: Molly Smullen, Senior at Emma Willard (M)
Interviewee: Yi Zhang – graduate PhD candidate at RPI (Y)
M: Yi, can you provide a short summary of the work you do at
CBIS?
Y: I study protein folding with NMR fluorescence and Saxs
under high pressure.
M: What does studying proteins entail?
Y: To study protein folding, you need to break the balance
of proteins in the unfolded versus folded states to understand what propels the
protein into its folded state. Whatever parameter you use to break this balance
is called a denaturant. Common denaturants are chemicals (such as urea),
pressure, or temperature. In my lab, we use high pressure as our denaturant,
because we believe it is a softer denaturant, and targets protein structure
locally. Only the cavity, where proteins are not perfectly packed, is targeted.
M: What are some different techniques for studying proteins?
Y: You should always use a wide range of biophysical methods
for studying proteins because they each reveal something different. For
example, Sexs provides information about the overall conformation of the
protein, and informs you of overall change in protein shape. Fluorescence is
also used to study the general form of proteins. NMR provides more detailed
information because its resolution is resolved to the atomic residue.
Therefore, you are provided with sequence-based information.
M: How did you become interested in this sort of work?
Y: I have always been interested in biology, since high
school, or even middle school. It was very natural to take on this research
path, and proteins are a hot topic right now. Also, the techniques you use in
protein studies are widely used, so you are trained for many different areas when you study proteins.
M: What implications does your research have in the scientific
community/the world?
Y: It’s always good to study protein folding mechanisms
because proteins are the major functional components of our bodies. Drug design
is also becoming more target-based on protein structures, so this research
could provide some insight and guidelines for future drug design.
M: What are your future
plans?
Y: I am planning on going to law school and becoming a
patent lawyer.
M: Why did you open your doors for an intern?
Y: I am a student, so I know what such an extracurricular
activity means to a student who is eager to learn an explore that interest. It
is my pleasure and honor to help introduce students to their passions. My
father and grandfather were also teachers, so it is good for me to share my
knowledge with others, especially younger students.
M: Is there anything you would like to add?
Y: Good luck. When you go to college, you should definitely
study, but don’t only study. Do some extracurricular activities, and not just
academic ones. Be social, develop interpersonal skills. College is a great time
for you to explore and grow into an adult. Study, but don’t be a nerd. Grow
into your own person, and have fun!
M: Thanks, Yi. I really appreciate it.
Y: You are welcome.
Subscribe to:
Comments (Atom)